
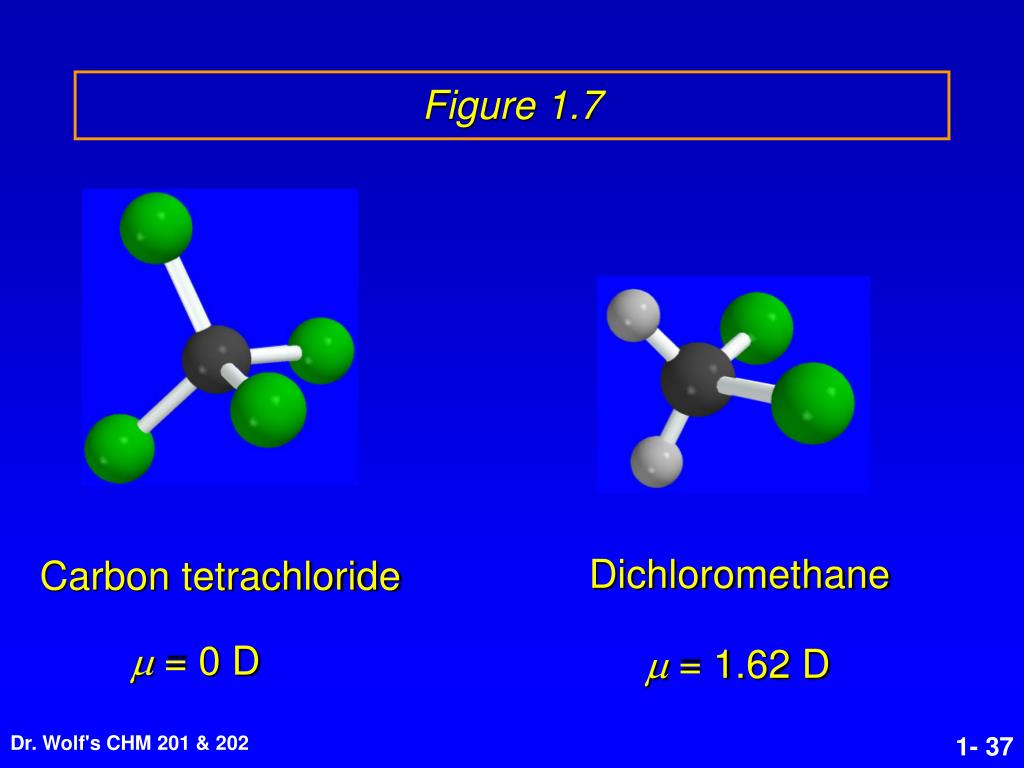
If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say “monoxide” rather than “monooxide” and “trioxide” rather than “troxide”). Normally, no prefix is added to the first element’s name if there is only one atom of the first element in a molecule. Table 4.1 “Numerical Prefixes for Naming Binary Covalent Compounds” lists these numerical prefixes. Give the correct formula and chemical name for the ionic compound: Ca2+ and CO32-Give the correct formula and chemical name for the ionic compound: Fe3+ and CO32. Determine the partial (or full) positive and negative charges if the bond has them. Which is of these is the correct chemical formula for carbon tetrachloride a. chain transfer agents like carbon tetrachloride, mercaptans etc.
#Carbon tetrachloride formula ionic or molecular free#
Characterize the C-C bond as nonpolar, polar covalent, or ionic. These ions or free radicals, then add to multiple bond of another monomer unit to. A system of numerical prefixes is used to specify the number of atoms in a molecule. Determine the molecular formula, Lewis structure, shape, polarity, and intermolecular force for carbon disulfide molecule. The second element is named by taking the stem of the element name and adding the suffix – ide. The first element in the formula is simply listed using the name of the element. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. For example, we have already seen CH 4, the molecular formula for methane. Numerical subscripts are used if there is more than one of a particular atom.
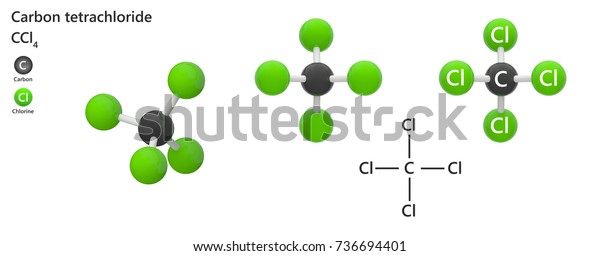
Thus, the compound formed from sodium and chlorine will be ionic (a. Compounds that involve a metal binding with either a non-metal will display ionic bonding. For each of the following questions, determine whether the compound is ionic or covalent and write the appropriate formula for it. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Then the other nonmetal symbols are listed. Covalent bonds form when two or more nonmetals combine. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H 2O is the prominent exception). Trending Questions What characteristic of minerals is not a characteristic of mineraloids? Can pollution cause earthquake? How many feet below sea level equals 10 Bar? What three words make up insolation? What are 5 facts about static electricity? Is an apple a compound or mixture? What are the main features of the lithosphere? How many earthquakes have occurred with 9.0? What is the shorthand notation for a compound? Which is least similar lead copper iron tin or brass? What season do earthquakes occur? Is there any connection between mount saint helens earthquake and the loma prieta earthquake? What are three ways the Hopi adapted to the dry climate of the southwest? What does sulfur dioxide indicate about a volcano? Which organisms fix nitrogen in aquatic ecosystems? When tectonic plates move it causes what in the earths crust? What theory states that the geologic forces and processes acting on the Earth today are the sames as those that have acted in the geologic past? What is the name of the compound S2O3? What is the freezing point of osmium? P waves and S waves are two kinds of seismic waves.The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules.


 0 kommentar(er)
0 kommentar(er)
